Page 31 - NCERT Science Class 10 English Medium
P. 31
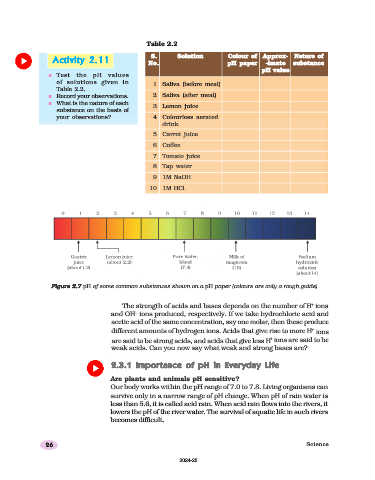
Table 2.2
S. Solution Colour of Approx- Nature of
Activity
Activity 2.11
Activity 2.11
Activity 2.11 No. pH paper -imate substance
Activity 2.112.11
pH value
n Test the pH values
of solutions given in
1 Saliva (before meal)
Table 2.2.
n Record your observations. 2 Saliva (after meal)
n What is the nature of each
3 Lemon juice
substance on the basis of
your observations? 4 Colourless aerated
drink
5 Carrot juice
6 Coffee
7 Tomato juice
8 Tap water
9 1M NaOH
10 1M HCl
Figure 2.7
Figure 2.7
Figure 2.7 pH of some common substances shown on a pH paper (colours are only a rough guide)
Figure 2.7
Figure 2.7
+
The strength of acids and bases depends on the number of H ions
and OH ions produced, respectively. If we take hydrochloric acid and
–
acetic acid of the same concentration, say one molar, then these produce
different amounts of hydrogen ions. Acids that give rise to more H ions
+
+
are said to be strong acids, and acids that give less H ions are said to be
weak acids. Can you now say what weak and strong bases are?
2.3.1
2.3.1 ImporImportance of pH in Evertance of pH in Everyday Lifeyday Life
2.3.1 Importance of pH in Everyday Life
Impor
2.3.1 Importance of pH in Evertance of pH in Everyday Lifeyday Life
2.3.1
Are plants and animals pH sensitive?
Our body works within the pH range of 7.0 to 7.8. Living organisms can
survive only in a narrow range of pH change. When pH of rain water is
less than 5.6, it is called acid rain. When acid rain flows into the rivers, it
lowers the pH of the river water. The survival of aquatic life in such rivers
becomes difficult.
26 Science
2024-25