Page 41 - NCERT Science Class 10 English Medium
P. 41
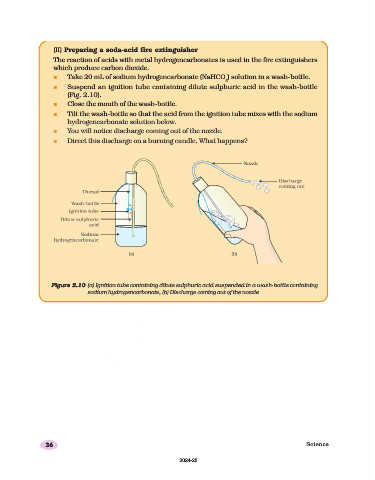
(II) Preparing a soda-acid fire extinguisher
The reaction of acids with metal hydrogencarbonates is used in the fire extinguishers
which produce carbon dioxide.
n Take 20 mL of sodium hydrogencarbonate (NaHCO ) solution in a wash-bottle.
3
n Suspend an ignition tube containing dilute sulphuric acid in the wash-bottle
(Fig. 2.10).
n Close the mouth of the wash-bottle.
n Tilt the wash-bottle so that the acid from the ignition tube mixes with the sodium
hydrogencarbonate solution below.
n You will notice discharge coming out of the nozzle.
n Direct this discharge on a burning candle. What happens?
Figure 2.10 (a) Ignition tube containing dilute sulphuric acid suspended in a wash-bottle containing
Figure 2.10
Figure 2.10
Figure 2.10
Figure 2.10
sodium hydrogencarbonate, (b) Discharge coming out of the nozzle
36 Science
2024-25