Page 16 - Understanding NCERT Science 09
P. 16
higher than that of solids. This is due to the We have observed that gases are highly
fact that in the liquid state, particles move compressible as compared to solids and
freely and have greater space between each liquids. The liquefied petroleum gas (LPG)
other as compared to particles in the solid cylinder that we get in our home for cooking
state. or the oxygen supplied to hospitals in
cylinders is compressed gas. Compressed
natural gas (CNG) is used as fuel these days
1.3.3 THE GASEOUS STATE
in vehicles. Due to its high compressibility,
Have you ever observed a balloon seller filling large volumes of a gas can be compressed
a large number of balloons from a single into a small cylinder and transported easily.
cylinder of gas? Enquire from him how many We come to know of what is being cooked
balloons is he able to fill from one cylinder. in the kitchen without even entering there,
Ask him which gas does he have in the cylinder. by the smell that reaches our nostrils. How
does this smell reach us? The particles of the
Activity _____________1.11 aroma of food mix with the particles of air
spread from the kitchen, reach us and even
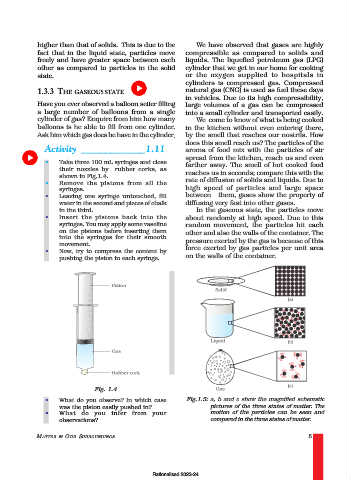
• Take three 100 mL syringes and close
farther away. The smell of hot cooked food
their nozzles by rubber corks, as reaches us in seconds; compare this with the
shown in Fig.1.4.
• Remove the pistons from all the rate of diffusion of solids and liquids. Due to
syringes. high speed of particles and large space
• Leaving one syringe untouched, fill between them, gases show the property of
water in the second and pieces of chalk diffusing very fast into other gases.
in the third. In the gaseous state, the particles move
• Insert the pistons back into the about randomly at high speed. Due to this
syringes. You may apply some vaseline random movement, the particles hit each
on the pistons before inserting them other and also the walls of the container. The
into the syringes for their smooth pressure exerted by the gas is because of this
movement. force exerted by gas particles per unit area
• Now, try to compress the content by
pushing the piston in each syringe. on the walls of the container.
Fig. 1.4
• What do you observe? In which case Fig.1.5: a, b and c show the magnified schematic
was the piston easily pushed in? pictures of the three states of matter. The
• What do you infer from your motion of the particles can be seen and
observations? compared in the three states of matter.
MATTER IN OUR SURROUNDINGS 5
Rationalised 2023-24