Page 54 - Understanding NCERT Science 09
P. 54
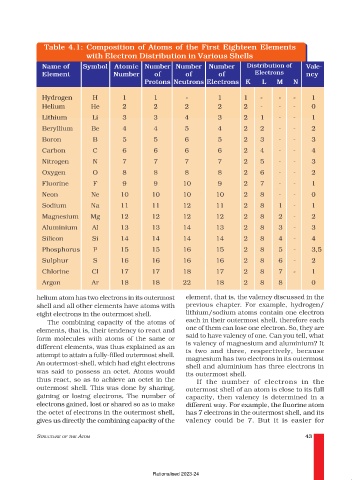
Table 4.1: Composition of Atoms of the First Eighteen Elements
with Electron Distribution in Various Shells
Name of Symbol Atomic Number Number Number Distribution of Vale-
Element Number of of of Electrons ncy
Protons Neutrons Electrons K L M N
Hydrogen H 1 1 - 1 1 - - - 1
Helium He 2 2 2 2 2 - - - 0
Lithium Li 3 3 4 3 2 1 - - 1
Beryllium Be 4 4 5 4 2 2 - - 2
Boron B 5 5 6 5 2 3 - - 3
Carbon C 6 6 6 6 2 4 - - 4
Nitrogen N 7 7 7 7 2 5 - - 3
Oxygen O 8 8 8 8 2 6 - - 2
Fluorine F 9 9 10 9 2 7 - - 1
Neon Ne 10 10 10 10 2 8 - - 0
Sodium Na 11 11 12 11 2 8 1 - 1
Magnesium Mg 12 12 12 12 2 8 2 - 2
Aluminium Al 13 13 14 13 2 8 3 - 3
Silicon Si 14 14 14 14 2 8 4 - 4
Phosphorus P 15 15 16 15 2 8 5 - 3,5
Sulphur S 16 16 16 16 2 8 6 - 2
Chlorine Cl 17 17 18 17 2 8 7 - 1
Argon Ar 18 18 22 18 2 8 8 0
helium atom has two electrons in its outermost element, that is, the valency discussed in the
shell and all other elements have atoms with previous chapter. For example, hydrogen/
eight electrons in the outermost shell. lithium/sodium atoms contain one electron
The combining capacity of the atoms of each in their outermost shell, therefore each
one of them can lose one electron. So, they are
elements, that is, their tendency to react and
form molecules with atoms of the same or said to have valency of one. Can you tell, what
is valency of magnesium and aluminium? It
different elements, was thus explained as an
is two and three, respectively, because
attempt to attain a fully-filled outermost shell.
magnesium has two electrons in its outermost
An outermost-shell, which had eight electrons
shell and aluminium has three electrons in
was said to possess an octet. Atoms would its outermost shell.
thus react, so as to achieve an octet in the If the number of electrons in the
outermost shell. This was done by sharing, outermost shell of an atom is close to its full
gaining or losing electrons. The number of capacity, then valency is determined in a
electrons gained, lost or shared so as to make different way. For example, the fluorine atom
the octet of electrons in the outermost shell, has 7 electrons in the outermost shell, and its
gives us directly the combining capacity of the valency could be 7. But it is easier for
STRUCTURE OF THE ATOM 43
Rationalised 2023-24