Page 9 - NCERT Science Class 10 English Medium
P. 9
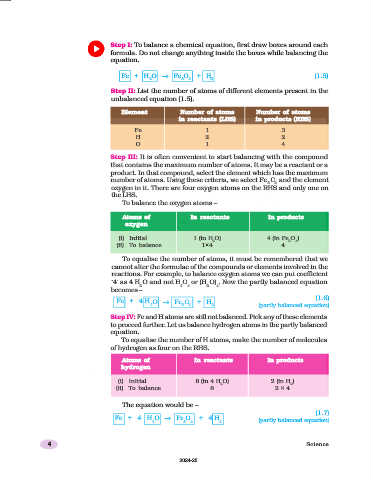
Step I: To balance a chemical equation, first draw boxes around each
formula. Do not change anything inside the boxes while balancing the
equation.
Fe + H O → Fe O + H (1.5)
2 3 4 2
Step II: List the number of atoms of different elements present in the
unbalanced equation (1.5).
Element Number of atoms Number of atoms
in reactants (LHS) in products (RHS)
Fe 1 3
H 2 2
O 1 4
Step III: It is often convenient to start balancing with the compound
that contains the maximum number of atoms. It may be a reactant or a
product. In that compound, select the element which has the maximum
number of atoms. Using these criteria, we select Fe O and the element
3 4
oxygen in it. There are four oxygen atoms on the RHS and only one on
the LHS.
To balance the oxygen atoms –
Atoms of In reactants In products
oxygen
(i) Initial 1 (in H O) 4 (in Fe O )
2 3 4
(ii) To balance 1×4 4
To equalise the number of atoms, it must be remembered that we
cannot alter the formulae of the compounds or elements involved in the
reactions. For example, to balance oxygen atoms we can put coefficient
‘4’ as 4 H O and not H O or (H O) . Now the partly balanced equation
2 2 4 2 4
becomes –
(1.6)
Fe + 4 H O → Fe O + H
2 3 4 2 (partly balanced equation)
Step IV: Fe and H atoms are still not balanced. Pick any of these elements
to proceed further. Let us balance hydrogen atoms in the partly balanced
equation.
To equalise the number of H atoms, make the number of molecules
of hydrogen as four on the RHS.
Atoms of In reactants In products
hydrogen
(i) Initial 8 (in 4 H O) 2 (in H )
2 2
(ii) To balance 8 2 × 4
The equation would be –
(1.7)
Fe + 4 H O → Fe O + 4 H
2 3 4 2 (partly balanced equation)
4 Science
2024-25