Page 32 - Understanding NCERT Science 09
P. 32
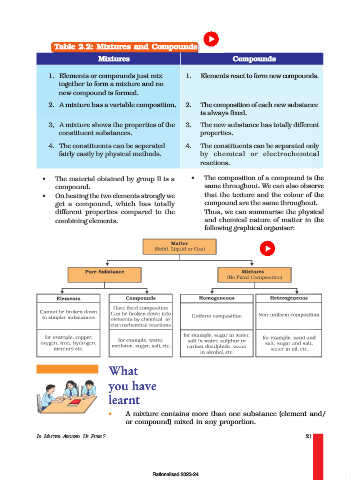
Table 2.2: Mixtures and Compounds
Mixtures Compounds
1. Elements or compounds just mix 1. Elements react to form new compounds.
together to form a mixture and no
new compound is formed.
2. A mixture has a variable composition. 2. The composition of each new substance
is always fixed.
3, A mixture shows the properties of the 3. The new substance has totally different
constituent substances. properties.
4. The constituents can be seperated 4. The constituents can be separated only
fairly easily by physical methods. by chemical or electrochemical
reactions.
• The material obtained by group II is a • The composition of a compound is the
compound. same throughout. We can also observe
• On heating the two elements strongly we that the texture and the colour of the
get a compound, which has totally compound are the same throughout.
different properties compared to the Thus, we can summarise the physical
combining elements. and chemical nature of matter in the
following graphical organiser:
What
you have
learnt
• A mixture contains more than one substance (element and/
or compound) mixed in any proportion.
IS MATTER AROUND US PURE? 21
Rationalised 2023-24