Page 43 - Understanding NCERT Science 09
P. 43
–
chloride ions (Cl ). A group of atoms carrying learn the symbols and combining capacity of
a charge is known as a polyatomic ion (Table the elements.
3.6). We shall learn more about the formation The combining power (or capacity) of an
of ions in Chapter 4.
element is known as its valency. Valency can
be used to find out how the atoms of an
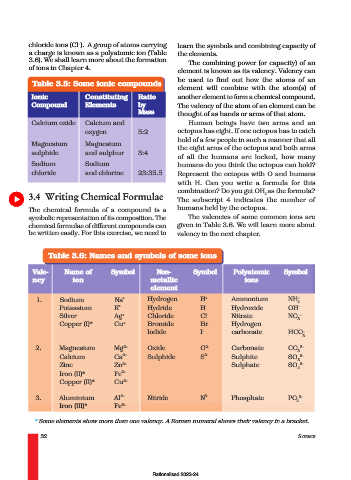
Table 3.5: Some ionic compounds
element will combine with the atom(s) of
Ionic Constituting Ratio another element to form a chemical compound.
Compound Elements by The valency of the atom of an element can be
Mass thought of as hands or arms of that atom.
Calcium oxide Calcium and Human beings have two arms and an
oxygen 5:2 octopus has eight. If one octopus has to catch
hold of a few people in such a manner that all
Magnesium Magnesium
the eight arms of the octopus and both arms
sulphide and sulphur 3:4
of all the humans are locked, how many
Sodium Sodium humans do you think the octopus can hold?
chloride and chlorine 23:35.5 Represent the octopus with O and humans
with H. Can you write a formula for this
combination? Do you get OH as the formula?
3.4 Writing Chemical Formulae The subscript 4 indicates the number of
4
The chemical formula of a compound is a humans held by the octopus.
symbolic representation of its composition. The The valencies of some common ions are
chemical formulae of different compounds can given in Table 3.6. We will learn more about
be written easily. For this exercise, we need to valency in the next chapter.
Table 3.6: Names and symbols of some ions
Vale- Name of Symbol Non- Symbol Polyatomic Symbol
ncy ion metallic ions
element
1. Sodium Na + Hydrogen H + Ammonium NH +
4
Potassium K + Hydride H - Hydroxide OH –
Silver Ag + Chloride Cl - Nitrate NO –
3
Copper (I)* Cu + Bromide Br - Hydrogen
Iodide I – carbonate HCO –
3
2. Magnesium Mg 2+ Oxide O 2- Carbonate CO 2–
3
Calcium Ca 2+ Sulphide S 2- Sulphite SO 2–
3
Zinc Zn 2+ Sulphate SO 2–
4
Iron (II)* Fe 2+
Copper (II)* Cu 2+
3. Aluminium Al 3+ Nitride N 3- Phosphate PO 3–
4
Iron (III)* Fe 3+
* Some elements show more than one valency. A Roman numeral shows their valency in a bracket.
32 SCIENCE
Rationalised 2023-24