Page 52 - NCERT Science Class 10 English Medium
P. 52
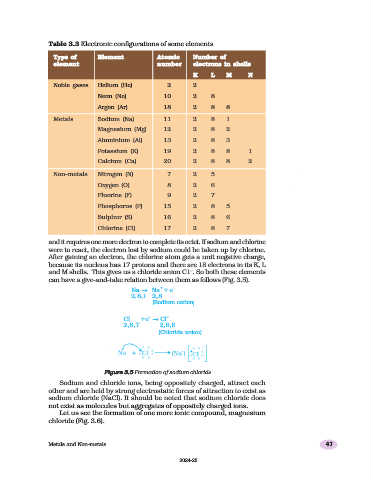
Table 3.3 Electronic configurations of some elements
Type of Element Atomic Number of
element number electrons in shells
K L M N
Noble gases Helium (He) 2 2
Neon (Ne) 10 2 8
Argon (Ar) 18 2 8 8
Metals Sodium (Na) 11 2 8 1
Magnesium (Mg) 12 2 8 2
Aluminium (Al) 13 2 8 3
Potassium (K) 19 2 8 8 1
Calcium (Ca) 20 2 8 8 2
Non-metals Nitrogen (N) 7 2 5
Oxygen (O) 8 2 6
Fluorine (F) 9 2 7
Phosphorus (P) 15 2 8 5
Sulphur (S) 16 2 8 6
Chlorine (Cl) 17 2 8 7
and it requires one more electron to complete its octet. If sodium and chlorine
were to react, the electron lost by sodium could be taken up by chlorine.
After gaining an electron, the chlorine atom gets a unit negative charge,
because its nucleus has 17 protons and there are 18 electrons in its K, L
–
and M shells. This gives us a chloride anion C1 . So both these elements
can have a give-and-take relation between them as follows (Fig. 3.5).
+
Na → Na + e –
2,8,1 2,8
(Sodium cation)
–
Cl +e → Cl –
2,8,7 2,8,8
(Chloride anion)
Figure 3.5
Figure 3.5
Figure 3.5
Figure 3.5
Figure 3.5 Formation of sodium chloride
Sodium and chloride ions, being oppositely charged, attract each
other and are held by strong electrostatic forces of attraction to exist as
sodium chloride (NaCl). It should be noted that sodium chloride does
not exist as molecules but aggregates of oppositely charged ions.
Let us see the formation of one more ionic compound, magnesium
chloride (Fig. 3.6).
Metals and Non-metals 47
2024-25