Page 48 - NCERT Science Class 10 English Medium
P. 48
Figure 3.3
Figure 3.3
Figure 3.3
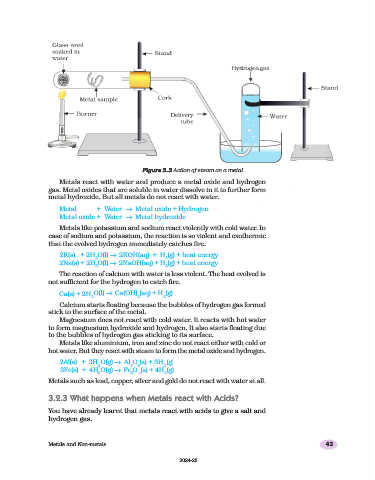
Figure 3.3 Action of steam on a metal
Figure 3.3
Metals react with water and produce a metal oxide and hydrogen
gas. Metal oxides that are soluble in water dissolve in it to further form
metal hydroxide. But all metals do not react with water.
Metal + Water → Metal oxide + Hydrogen
Metal oxide + Water → Metal hydroxide
Metals like potassium and sodium react violently with cold water. In
case of sodium and potassium, the reaction is so violent and exothermic
that the evolved hydrogen immediately catches fire.
2K(s) + 2H O(l) → 2KOH(aq) + H (g) + heat energy
2 2
2Na(s) + 2H O(l) → 2NaOH(aq) + H (g) + heat energy
2 2
The reaction of calcium with water is less violent. The heat evolved is
not sufficient for the hydrogen to catch fire.
Ca(s) + 2H O(l) → Ca(OH) (aq) + H (g)
2 2 2
Calcium starts floating because the bubbles of hydrogen gas formed
stick to the surface of the metal.
Magnesium does not react with cold water. It reacts with hot water
to form magnesium hydroxide and hydrogen. It also starts floating due
to the bubbles of hydrogen gas sticking to its surface.
Metals like aluminium, iron and zinc do not react either with cold or
hot water. But they react with steam to form the metal oxide and hydrogen.
2Al(s) + 3H O(g) → Al O (s) + 3H (g)
2 2 3 2
3Fe(s) + 4H O(g) → Fe O (s) + 4H (g)
2 3 4 2
Metals such as lead, copper, silver and gold do not react with water at all.
3.2.3 What happens when Metals react with Acids?
You have already learnt that metals react with acids to give a salt and
hydrogen gas.
Metals and Non-metals 43
2024-25