Page 69 - NCERT Science Class 10 English Medium
P. 69
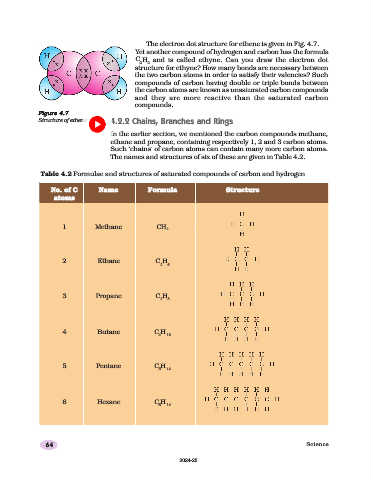
The electron dot structure for ethene is given in Fig. 4.7.
Yet another compound of hydrogen and carbon has the formula
C H and is called ethyne. Can you draw the electron dot
2 2
structure for ethyne? How many bonds are necessary between
the two carbon atoms in order to satisfy their valencies? Such
compounds of carbon having double or triple bonds between
the carbon atoms are known as unsaturated carbon compounds
and they are more reactive than the saturated carbon
compounds.
Figure 4.7
Figure 4.7
Figure 4.7
Figure 4.7
Figure 4.7
Structure of ethene 4.2.2 Chains, Branches and Rings
In the earlier section, we mentioned the carbon compounds methane,
ethane and propane, containing respectively 1, 2 and 3 carbon atoms.
Such ‘chains’ of carbon atoms can contain many more carbon atoms.
The names and structures of six of these are given in Table 4.2.
Table 4.2 Formulae and structures of saturated compounds of carbon and hydrogen
No. of C Name Formula Structure
atoms
1 Methane CH
4
2 Ethane C H
2 6
3 Propane C H
3 8
4 Butane C H
4 10
5 Pentane C H
5 12
6 Hexane C H
6 14
64 Science
2024-25