Page 70 - NCERT Science Class 10 English Medium
P. 70
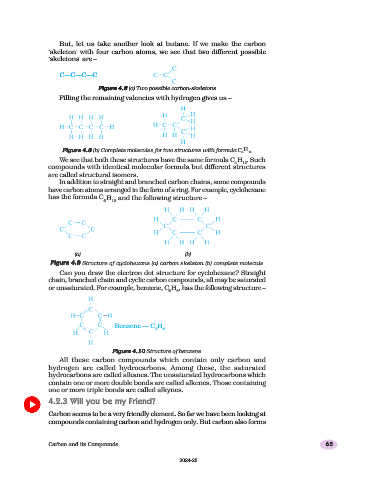
But, let us take another look at butane. If we make the carbon
‘skeleton’ with four carbon atoms, we see that two different possible
‘skeletons’ are –
C—C—C—C
Figure 4.8
Figure 4.8
Figure 4.8 (a) Two possible carbon-skeletons
Figure 4.8
Figure 4.8
Filling the remaining valencies with hydrogen gives us –
Figure 4.8
Figure 4.8
Figure 4.8 (b) Complete molecules for two structures with formula C H
Figure 4.8
Figure 4.8
4 10
We see that both these structures have the same formula C H . Such
4 10
compounds with identical molecular formula but different structures
are called structural isomers.
In addition to straight and branched carbon chains, some compounds
have carbon atoms arranged in the form of a ring. For example, cyclohexane
has the formula C H and the following structure –
6 12
(a) (b)
Figure 4.9 Structure of cyclohexane (a) carbon skeleton (b) complete molecule
Figure 4.9
Figure 4.9
Figure 4.9
Figure 4.9
Can you draw the electron dot structure for cyclohexane? Straight
chain, branched chain and cyclic carbon compounds, all may be saturated
or unsaturated. For example, benzene, C H , has the following structure –
6 6
Benzene — C H
6 6
Figure 4.10 Structure of benzene
Figure 4.10
Figure 4.10
Figure 4.10
Figure 4.10
All these carbon compounds which contain only carbon and
hydrogen are called hydrocarbons. Among these, the saturated
hydrocarbons are called alkanes. The unsaturated hydrocarbons which
contain one or more double bonds are called alkenes. Those containing
one or more triple bonds are called alkynes.
4.2.3 Will you be my Friend?
Carbon seems to be a very friendly element. So far we have been looking at
compounds containing carbon and hydrogen only. But carbon also forms
Carbon and its Compounds 65
2024-25